Our Solution: T-Guard
T-Guard is a novel medicine with potential for the treatment of certain life-threatening immune conditions, such as several severe autoimmune diseases and transplant-related rejection. T-Guard had been clinically evaluated for treatment of steroid-resistant acute graft-versus-host disease (aGVHD), a life-threatening immunological condition that often develops in patients following hematopoietic stem cell transplantation.
T-Guard consists of a unique combination of two toxin-conjugated monoclonal antibodies, so-called immunotoxins, that target the CD3 and CD7 molecules on T cells and NK cells. Preclinical and clinical testing showed that T-Guard can swiftly reset the immune system in patients.
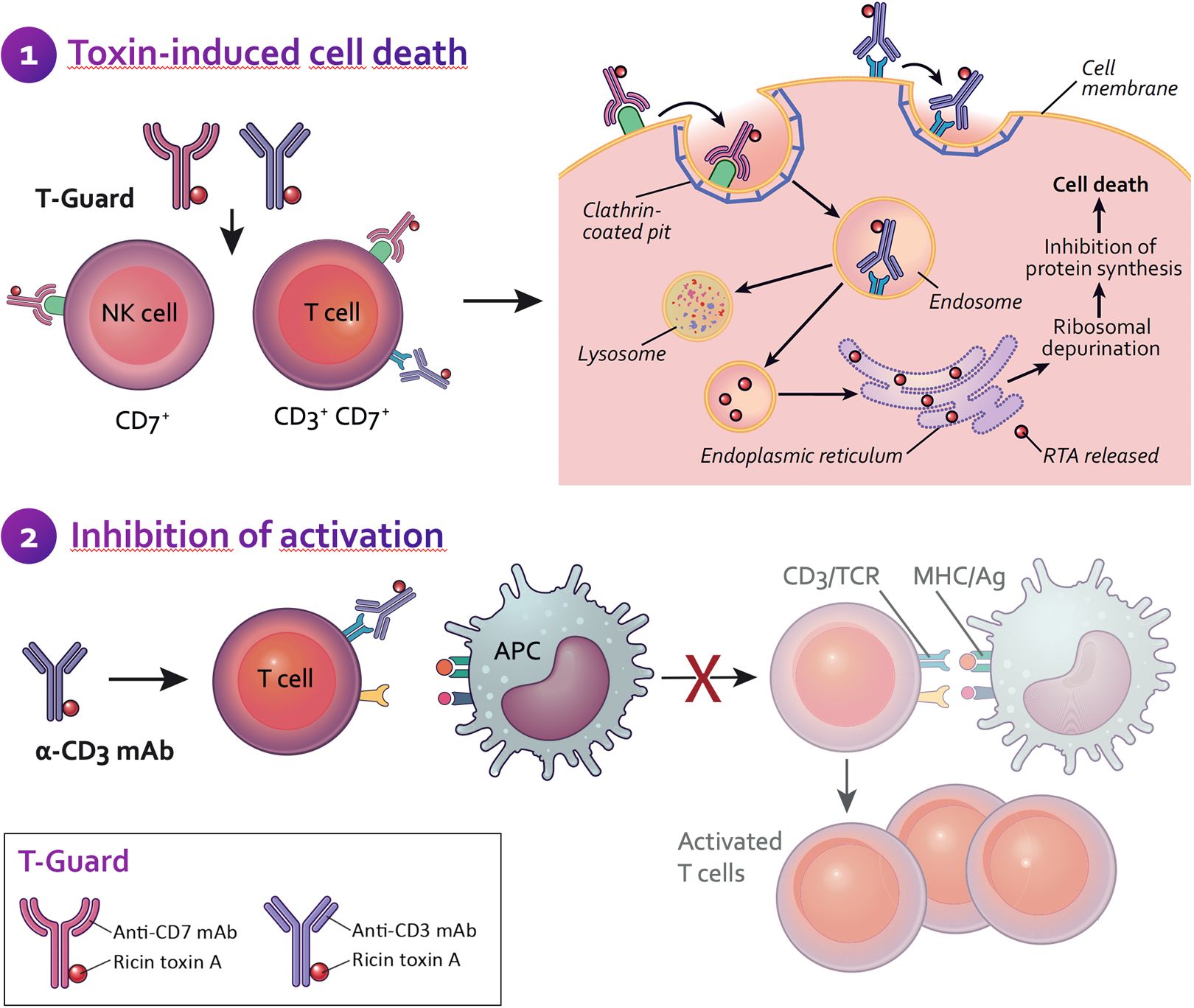
After injection into the body, T-Guard specifically identifies and eliminates mature T cells and NK cells, with high preference for activated T cells (the troublemakers). Each of the two antibodies in T-Guard is conjugated to an toxin that prevents the activated T cells from making new proteins, thereby inducing programmed cell death (apoptosis) with minimal treatment-related side effects. T-Guard’s brief, targeted action appears to limit the patient’s vulnerability to opportunistic infection whereas the commonly applied broad immunosuppression exacerbates this complication.
What makes T-Guard special?
The particular combination of immunotoxins used to construct T-Guard blends synergistic cytotoxicity and narrow specificity via multiple gentle mechanisms of action. Although the general principle behind immunotoxins as described above is not new, the particular combination of the two immunotoxins selected for T-Guard offer compelling differentiating features.
Synergistic cytotoxicity
The combination of half a dose of the anti-CD3 and anti-CD7 immunotoxins in T-Guard has been shown in pre-clinical testing to be more effective in eliminating T cells than a full dose of either immunotoxin alone. Simultaneous targeting via two distinct antigens appears to reduce the chance of T cells escaping treatment. Equally importantly, dividing the toxic payload over two different immunotoxins appears to reduce side effects. These features seem to increase the therapeutic window for T-Guard as compared to other immunotoxins using the same toxic payload and that have been clinically tested as individual compounds.
Multiple mechanisms for gentle T cell elimination
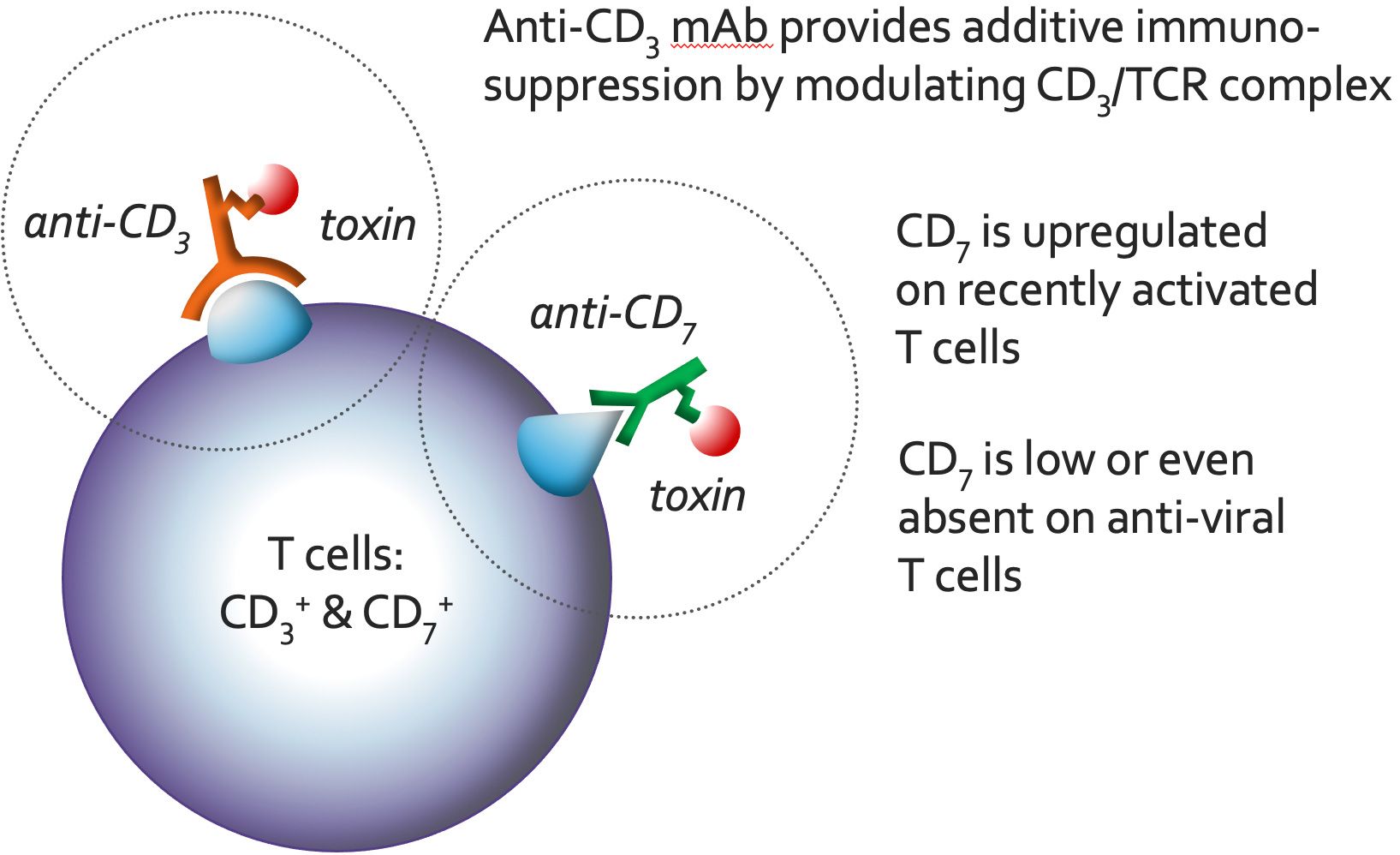
Both the anti-CD3 and anti-CD7 mAbs deliver the toxin to the inside of T cells and NK cells, resulting in the elimination of these cells by protein synthesis inhibition. Additionally, the binding of the anti-CD3 mAb to the T cell receptor/CD3 complex (TCR/CD3) results in an immunosuppressive effect through modulation/blocking of the TRC/CD3 and by activation-induced cell death (AICD). Both of these mechanisms enable a ‘gentle’ (non-inflammatory) elimination of T cells through apoptosis. Also, the particular anti-CD3 mAb selected for T-Guard does not stimulate T cells. These characteristics significantly reduce the occurrence of cytokine release syndrome (CRS), a potentially life-threatening complication frequently associated with alternative immunosuppressants targeting the TCR/CD3.
Narrow specificity and short half-life
T-Guard has a narrow specificity, directed only against mature T cells and NK cells. Importantly, recently activated T cells have been shown to be about 35 times more vulnerable to T-Guard than non-activated T cells.
It is thought that T-Guard may stop the pathologic immune response while generally sparing the resting T cell pool, which is able to counter future potentially dangerous infections. The elimination of NK cells is thought to be beneficial as these cells could aggravate the severity of certain immune conditions.
T-Guard is administered as four infusions in a treatment course of just one-week. Due to its short half-life - about nine hours - T-Guard is rapidly washed out after the last infusion, allowing the swift restoration of the T cell compartment with newly formed (non-disease causing) cells.
Due to the above characteristics, T-Guard compares favorably to alternative therapies consisting of (monoclonal) antibodies that cause depletion of T cells and/or other immune cells, or biologics and small molecule inhibitors that induce a functional suppression of the immune system resulting in an often prolonged and rather broad immunosuppression.
T-Guard may therefore change the treatment paradigm of severe autoimmune diseases in that a short and targeted course of treatment may induce lasting remissions of this debilitating disease while allowing for a swift restoration of a functional immune system.
Scientific Rationale
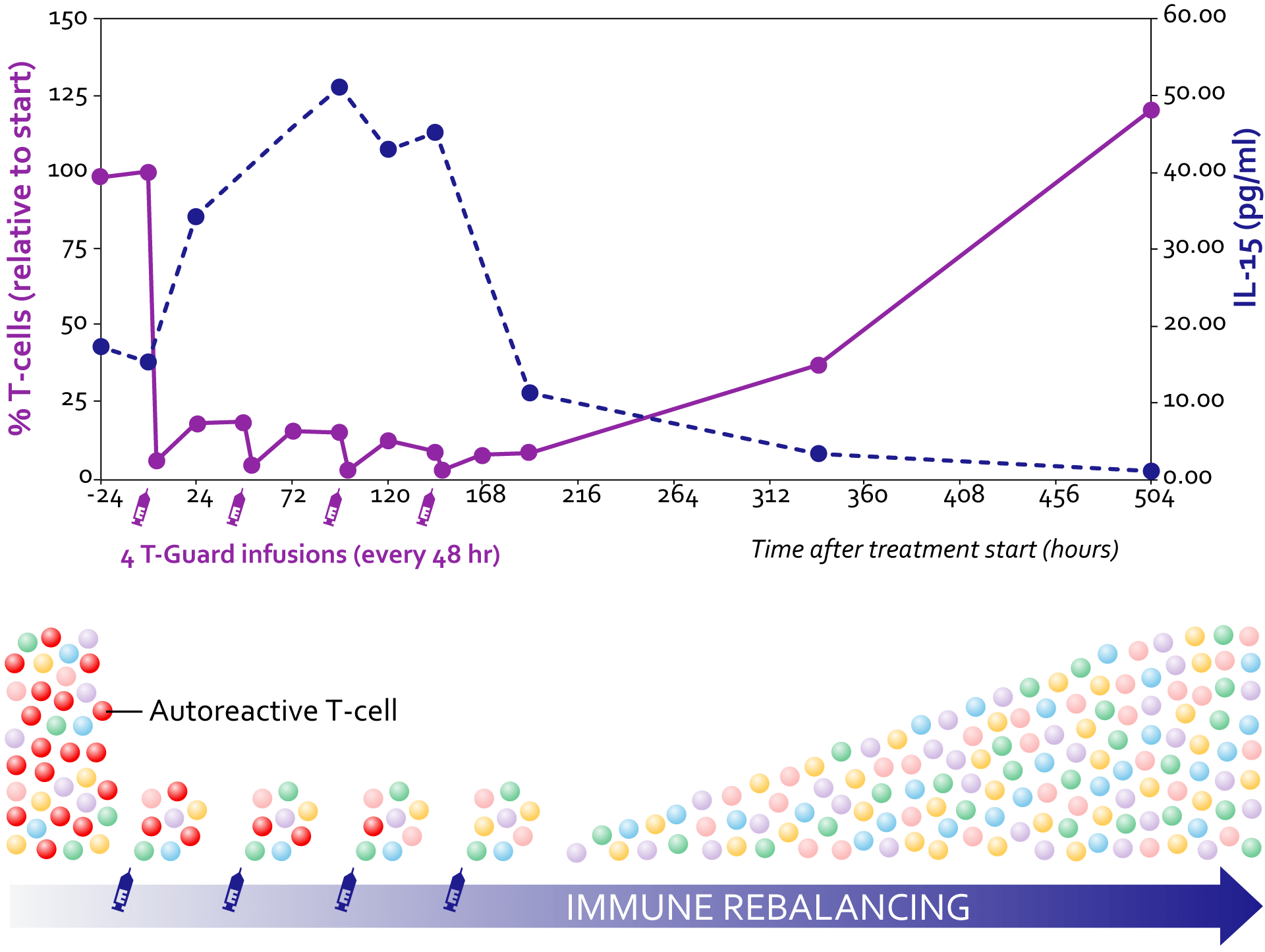
T-Guard's mechanism of action is based on a rebalancing of the immune system to a more tolerogenic state with a one-week treatment consisting of 4 infusions. T-Guard effectively depletes potentially pathogenic T and NK cells in vivo (having a higher membrane expression of the CD7 target antigen), while relatively sparing their non-activated counterparts. T cell compartment is swiftly repopulated with non-activated T cells that escaped treatment. Recurring T cells show a diverse T cell receptor repertoire.
1 week of T-Guard treament induced durable complete responses in a large fraction of patients with sever steroidrefractory acute GVHD.
Depletion of circulating T cells increases IL-15 levels, which in turn promote homeostatic T cell proliferation.
Preclinical Proof of Concept
T-Guard specifically and efficiently depleted T and NK cells in disease-affected tissues and blood derived from SSc patients. Moreover, T-Guard was also capable of strongly reducing fibroblast contraction that is characteristic for SSc by induced by incubating fibroblasts with PHA-activated PBMCs in a 3D fibrosis model.
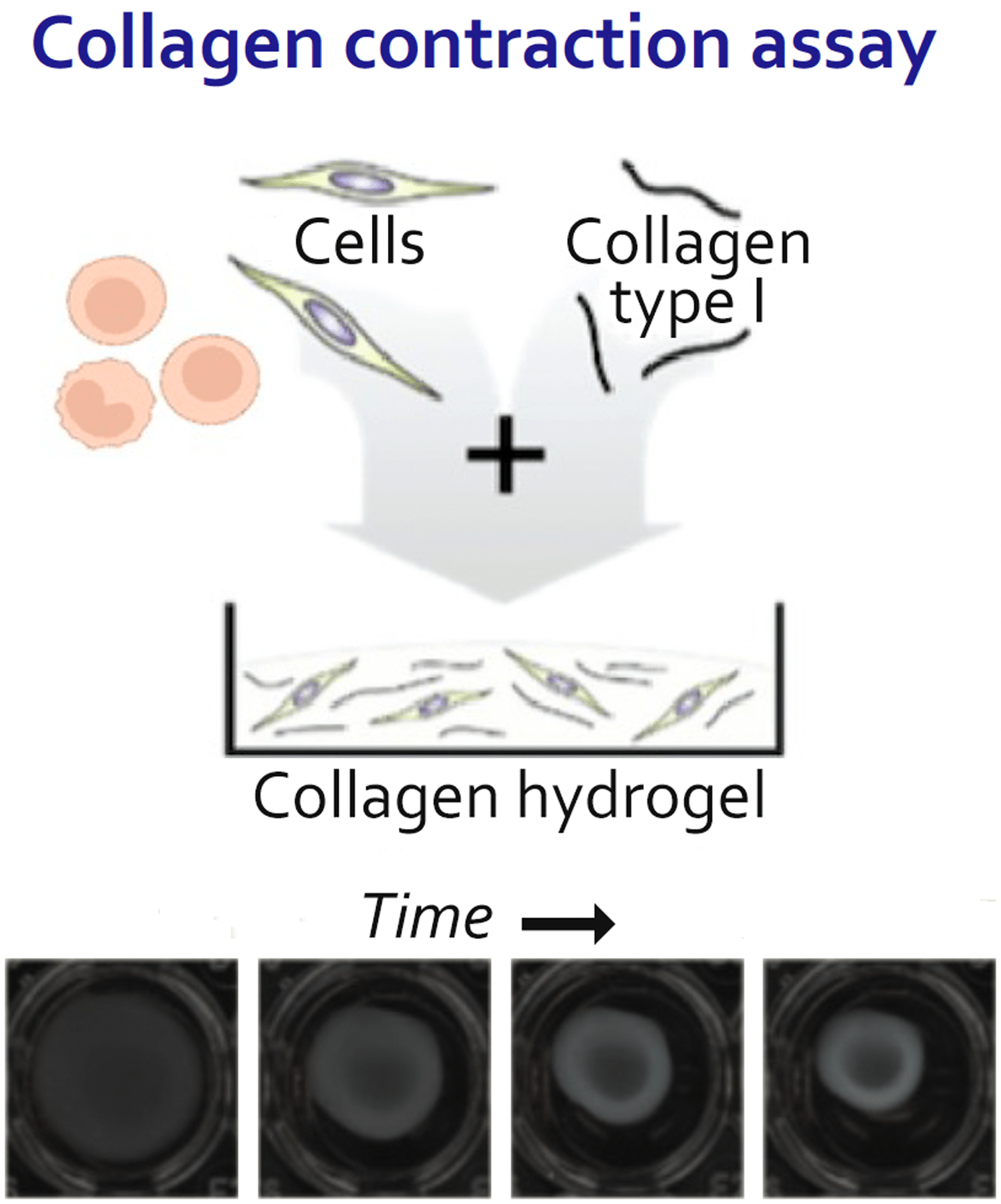
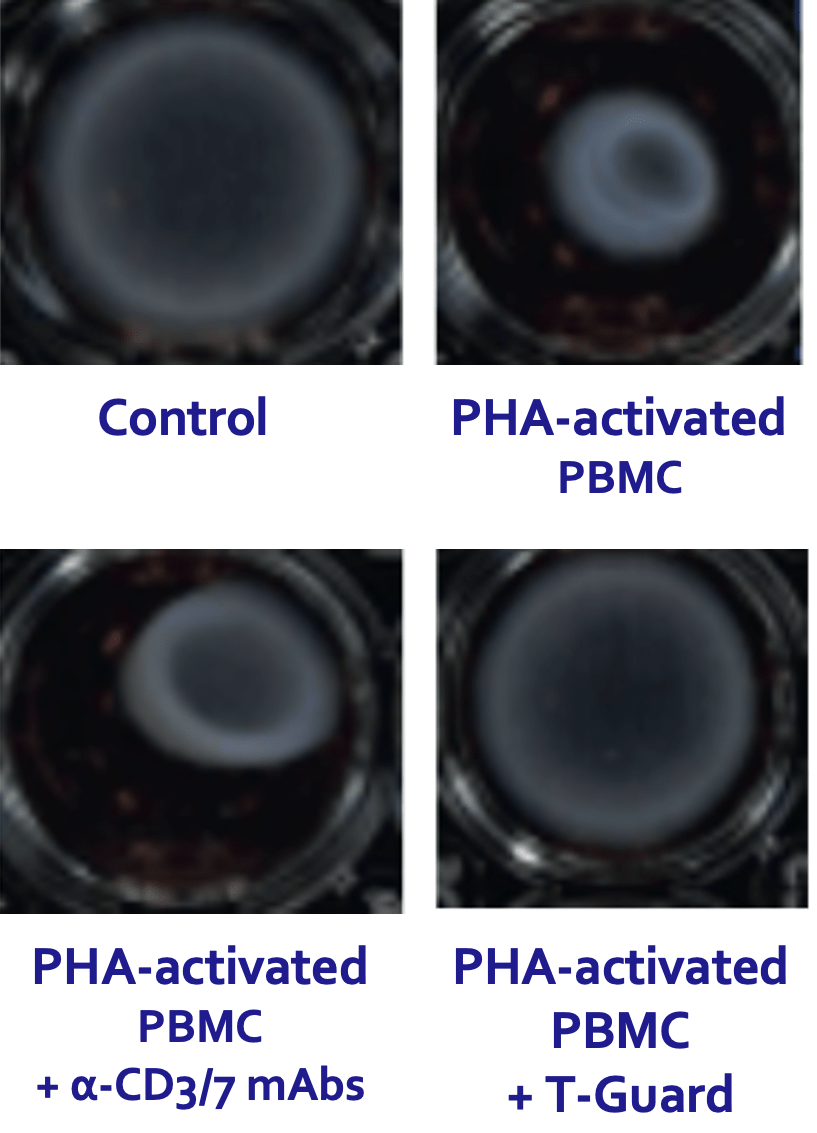
(Papadimitriou TI, et al. Ann Rheum Dis 2024;83:488–498)